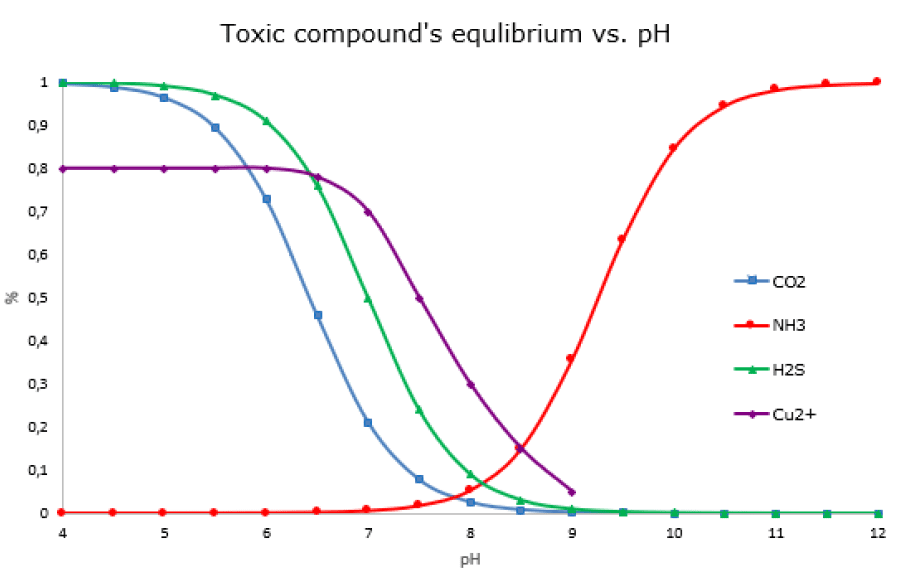
pH-management in a fish farm
pH affects the toxicity of substances in water. A typical pH-drop leads to a two- to threefold increase in the level of toxic free CO2, initiates the formation of H2S, and increases the toxicity of several metals, including copper (Cu2+). Conversely, an increase in pH-value will raise the concentration of unionized ammonia (NH3), which is also toxic to fish. Therefore, regulation of alkalinity and pH is an essential tool for controlling the toxicity of the water’s components.
Fill out the form to read more: